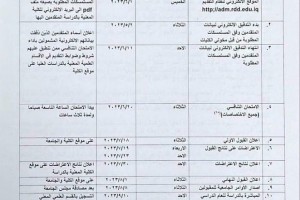
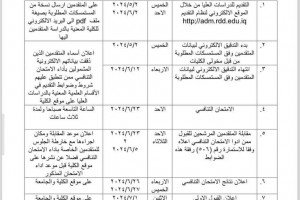
A Research team from the Department of Chemistry, University of Basrah ( Dr. Ali A. A. Al-Riyahee, Diyar Muhammad Ali Murad, and Ashwaq Abood Shenta) have published their scientific research about the electrothermal application of new complexes of benzamide in an International journal (Scopus Q3).
In this study, a new benzoylthiourea derivative was prepared:
[3,4-dichloro-N-((5-chloropyridin-2-yl)carbamothioyl)benzamide] ligand (L) and a series of complexes of copper (II), cobalt (II), nickel (II) and zinc (II). The as-prepared ligands and complexes were diagnosed by various techniques such as micro-elemental analysis, magnetic susceptibility, conductivity measurements, solubility test, mass spectra, infrared, UV-UV, 1H, 13C nuclear magnetic resonance spectroscopy. The spectroscopic techniques of these complexes showed that the free ligand L has a strong geometric preference for the planar square shape. Cyclic voltammetry studies show that Cu(II) complexes give a quasi-reversible, one-metal-based reduction process for electron transfer due to the Cu(II)/Cu(I) redox couple ) while two consecutive irreversible cascading processes are observed in the periodic voltammograms of cobalt(II) and Nickel(II) complexes due to the M(II)/M(I) and M(III)/M(II) redox pairs. This indicates that the free thiourea bond stabilizes copper (II) and copper (I) ions in their complexes while nickel (I) and cobalt (I) are unstable in the CH3CN solvent. Electrochemical methods of copper complexes indicate a semi-reversible process with low redox potential and high stability of Cu(II) and Cu(I).