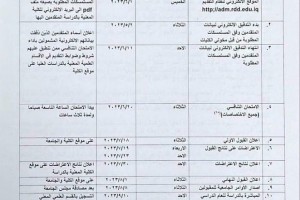
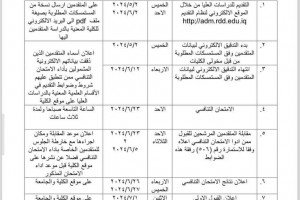
The University of Basrah hosted a master thesis defense “ Spectrophotometric method and conductometric titration for the determination of paracetamol and chlorpheniramine maleate in their pharmaceutical preparations:.
The presenter (Esraa Sabah Khewish) explained that the most important objectives of this study are:
1. Development of a simple and sensitive spectrophotometric method for the determination of paracetamol and chlorpheniramine maleate by oxidative coupling with the reagent ortho-aminophenol and2,3-dihydroxynaphthalene in the presence of N-bromosuccinimide in an acidic medium, both in its pure form and in its pharmaceutical preparations.
2. Development of a simple and sensitive method for the determination of chlorpheniramine maleate by measuring the electrical conductivity of chlorpheniramine maleate with sodium hydroxide reagent, both in its pure form and in its pharmaceutical preparations.(
The results were reached. The results showed that the method had a high degree of accuracy and compatibility, as the recovery value was 94.2% - 101.4% and the relative standard deviation value was less than 1.0%. As for the drug Chlorpheniramine Maleate, it gave the highest absorption at a wavelength of 360 nm, and followed Beer's law in the concentration range of -2-26 µg/ml. The molar absorptivity value was 6567.12 L mol cm, the Sandell's significance value was 0.06 µg/cm, the detection limit was 0.375 µg ml−1, and the quantum limit was 1.315 µg ml−1. The method had a high degree of accuracy and compatibility, as the recovery value was 95.35% - 103.7% and the relative standard deviation value was less than 1.0%. .
The study recommended to further study of the NMR and MS spectra of oxidative coupling complexes in various polar and nonpolar solvents. Also, to prepare of a new series of derivatives of amino and aminophenolic compounds and preparation of oxidative coupling complexes with various pharmaceutical compounds.